Can you imagine a scenario where a surgical
instrument used during an operation had microorganisms that cause issues to the
patient after the surgery? Pharmaceuticals and medical devices need to be
confidently sterile. They will be used in an environment where even a small
amount of contamination can have significant consequences. Sterility tests ensure
that no microorganisms are present in the product that is being used. But what
is the process for achieving this level of sterility assurance?
Firstly, we perform a Method Suitability Test.
It evaluates whether the testing method is appropriate and effective for
detecting if microorganisms are able to grow in the sample. Initially, it is important
to prepare the laboratory environment by making sure all the glassware and
containers involved are thoroughly cleaned and sterilized. Once we have all the
tools, we prepare and sterilize the culture media and phosphate buffer solution
(PBS) in accordance with manufacturer guidelines. For those who do not know,
culture media is a medium (solid or liquid) that contains essential nutrients to
promote microorganism growth. We use two different types of media to perform
the method suitability test according to the USP 71: Soybean-Casein Digest Medium
(tryptic soy broth-TSB) and Fluid thioglycolate medium (FTM).
We also need to add bacteria to see if there is growth. Therefore, we prepare bacterial
suspensions, and we dilute them using PBS by serial dilution until we achieve a
concentration of less than 100 Colony Forming Units (CFU).
Upon
the completion of the 14-day observation period, the sterility test outcome is
determined. If no signs of microbial growth are observed, the sample
successfully passes the sterility test and that’s how you make sure that
surgical instruments used on patients are sterile
But wait, what are Serial dilutions? In
microbiology, we gradually dilute liquids with lots of bacteria in them. Think
of it like adding water to juice step by step until it's just right. We take a
tiny bit of the original liquid, mix it with water (PBS in our case), then take
a bit of that mixture and mix it with more water (PBS) and this makes the
bacteria spread out, and thus, suitable for the study. This helps us make the
counting of bacteria easier because it can reduce the CFU count from 10,000 to less
than 100, which is required by the standard for sterility testing.
Let’s continue. Now, this diluted
suspension of less than 100 CFUs is then introduced into sterile containers
with the sample and the media. However, we cannot forget about the positive and
negative controls; they help us prove that the results are reliable, and not
influenced by other variables or factors.
For example, the positive control usually
includes culture media and the bacteria used to ensure that the conditions are
suitable for the growth of microorganisms. If the positive control doesn't
yield the expected results, it indicates potential issues with either the
culture media preparation, incubation conditions, or bacterial culture
viability. On the other hand, the negative control only includes the culture
media. If the negative control shows no change, it indicates that the culture
media is sterile and does not have any contaminants. Therefore, the effects
observed in the experimental groups are attributed solely to the presence of
the microorganisms or substances under investigation.
Once we have all the samples ready, they
are subjected to an incubation period of no more than 5 days, at optimal growth
temperature according to the bacteria used (TSB at 20-25 °C and FTM
between 30-35 °C). We monitor the incubation daily with careful documentation
recording. The final assessment criteria go as follows: if you do not observe the
growth of microorganisms that look like the positive control; then the sample contains
antimicrobial activity and the process needs to be changed before continuing
with the sterility test. However, if you observe visible growth of
microorganisms in the test sample that looks like the positive control sample, then
the product being tested doesn't show any antimicrobial activity in the given
experimental conditions. Thus, the sterility test can continue!
Once our method suitability test successfully
meets the defined acceptance criteria, we are ready to initiate the sterility
testing phase using the direct immersion method. This approach involves the
careful placement of the sample within an appropriate container, followed by
the introduction of culture media. The culture media of choice in this context
are the TSB and the FTM. It's important to note
that the volume of the liquid samples must not exceed 10% of the total culture
medium volume according to USP 71.
We use this specific culture media due to
their established efficacy for sterility testing. TSB promotes
the growth of fungi and aerobic bacteria, including microorganisms such as Aspergillus
brasiliensis, Bacillus subtilis, and Candida albicans. In
contrast, FTM serves as a medium of choice for the growth of aerobic and anaerobic
bacteria, such as Pseudomonas aeruginosa, Staphylococcus aureus,
and Clostridium sporogenes.
With the media and samples aseptically placed
within their respective containers, the next step is an incubation period of 14
days. The incubation temperatures are precisely regulated, with the TSB media
maintained at 20-25 °C,
while the FTM is incubated within the range of 30-35 °C. For each type of culture medium
employed, a negative control is also incubated, consisting only of a sterile
culture medium.
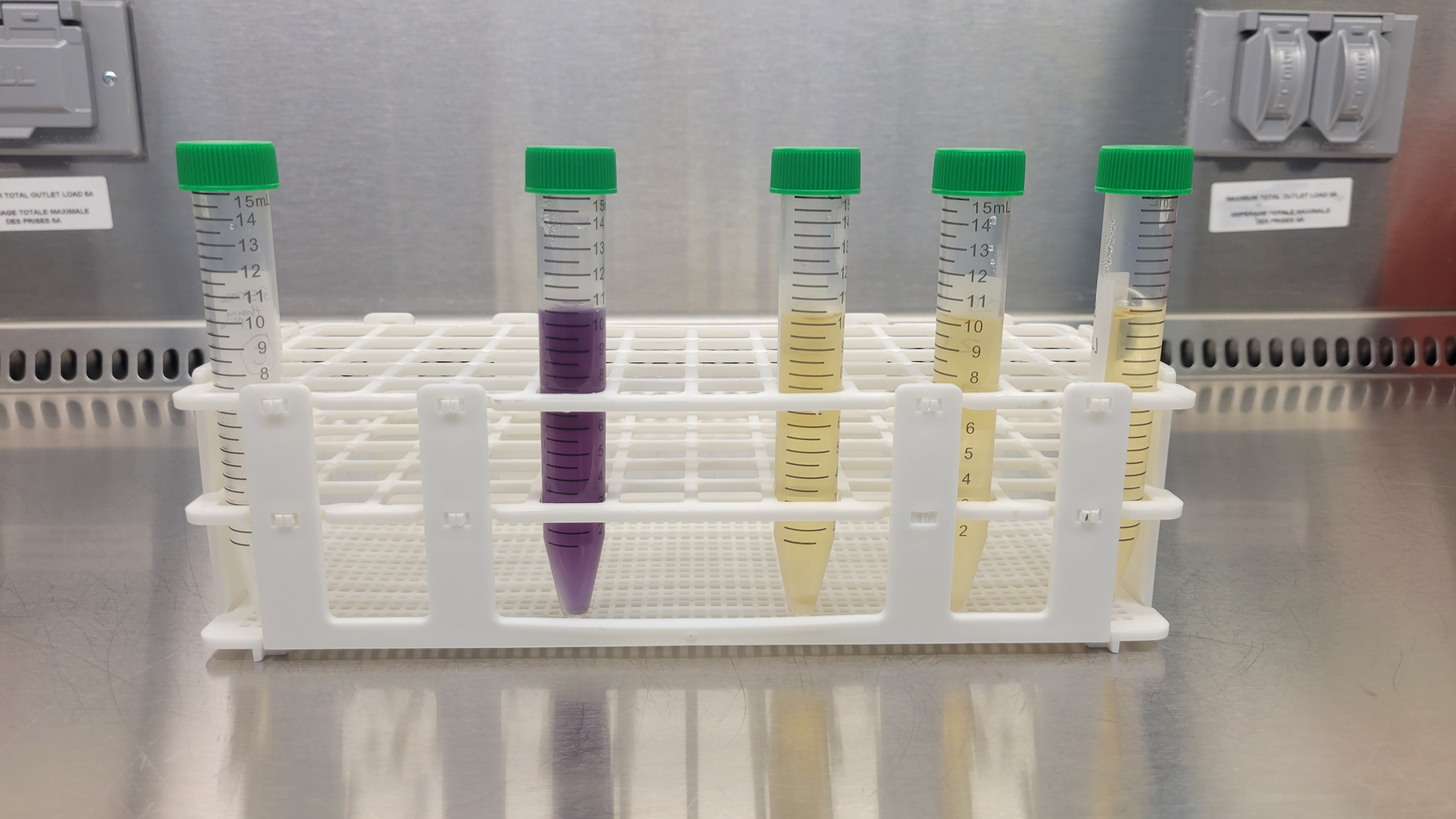
Throughout the 14-day incubation period, daily
visual observations of the media are conducted, and results are recorded.
Growth indicators encompass a variety of factors, including turbidity, changes
in odor, alterations in color, the appearance of pellicles, sediment formation,
and flocculation.

My SteriLabs Experience as an Interdisciplinary Biochemistry and Microbiology Intern. Blog post by Natasha Tal.
September 22, 2025
Microbiologist / Study Lead (Sterilization, Sterility Testing & QMS) — 24-Month Term
September 18, 2025
Media fill testing: Ensuring Aseptic Processing for Client Projects and Licence Applications. Blog Post by Natasha Tal
August 22, 2025