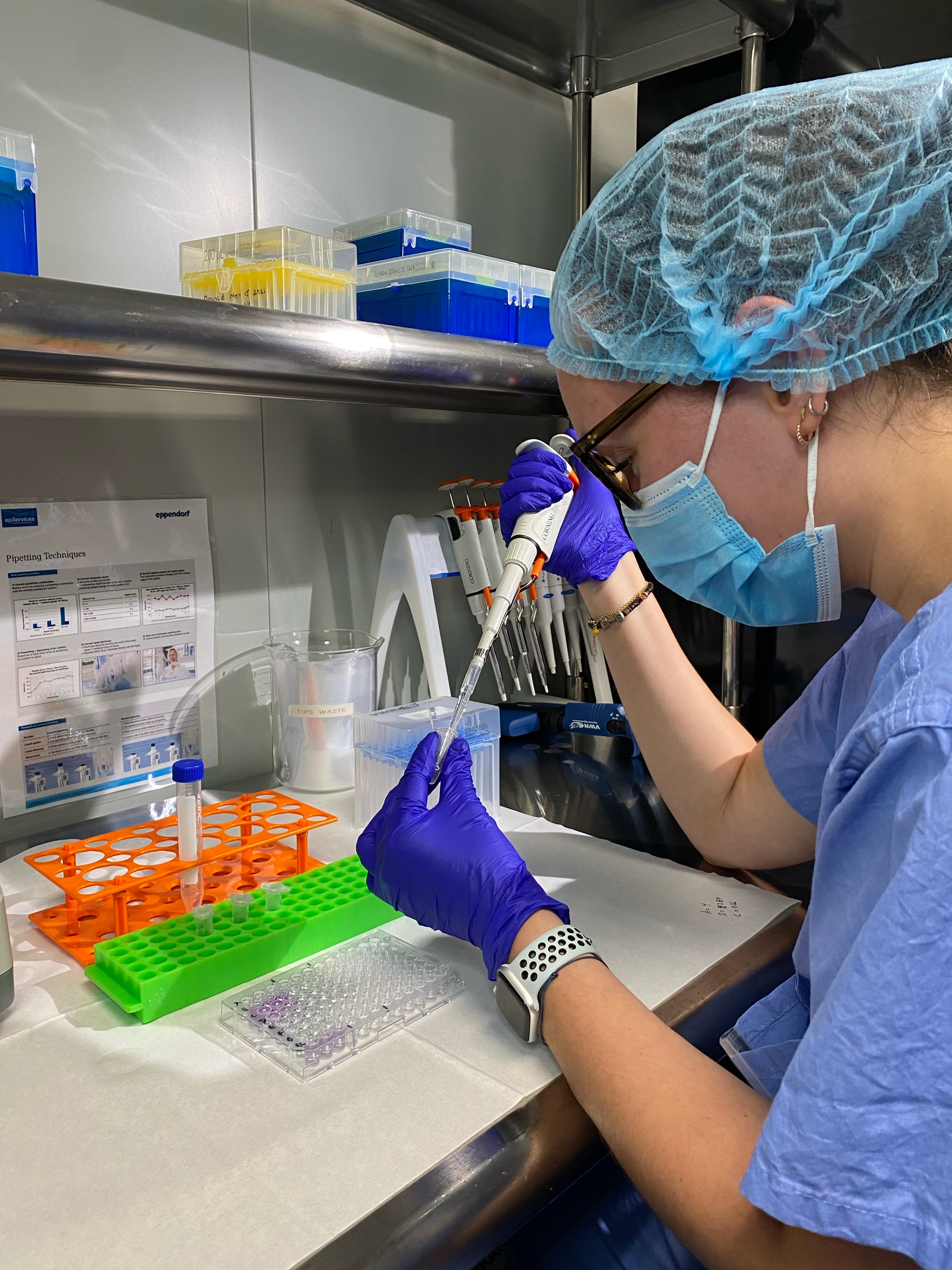
I’ve had the pleasure of working at SteriLabs as a biochemistry and microbiology lab intern. This has been an incredible opportunity that has allowed me to experience the unique environment of an industry lab, learn from experienced professionals, and gain hands-on exposure in
Read More
We’re adding an experienced Microbiologist / Study Lead for a 24-month term to lead sterilization validations, own sterility testing deliverables, support biochemistry cleaning-assay work, and help maintain our ISO 17025 QMS
Read More
Running a lab doesn’t just mean performing experiments and getting results. One of the many important aspects of being a properly functioning lab includes verifying the performance of lab procedures, and staying up-to-date with regulatory affairs. One of the many ways SteriLabs
Read More
As healthcare increasingly shifts beyond hospital walls, more patients are managing their health from the comfort of home. From blood pressure monitors to complex respiratory devices, home-use medical technologies are becoming essential tools in everyday life. But designing
Read More
This hands-on, on-site internship offers valuable real-world experience in a cutting-edge laboratory setting. You’ll work alongside industry experts, contributing to critical testing, validation, and R&D projects in medical device reprocessing
Read More
This hands-on, on-site internship provides real-world experience in a cutting-edge microbiology laboratory. You’ll work alongside industry experts, contributing to testing, validation, and regulatory compliance projects that impact healthcare worldwide
Read More
Ensuring that a medical device can be effectively cleaned, disinfected, and sterilized is a critical step in its development and regulatory approval process. However, many MedTech companies struggle to determine the optimal time to engage a medical device testing and validation
Read More
Steam sterilization, also known as autoclaving, is one of the most reliable and widely used methods for sterilizing medical devices. Whether it’s surgical instruments, implants, or reusable medical tools, ensuring that these devices are completely free from microorganisms is
Read More
Discover how SteriLabs has become a cornerstone for Canadian medical device manufacturers, offering world-class microbiology and analytical testing services. From reducing reliance on overseas labs to expanding ISO 17025 accreditation, this feature highlights SteriLabs’
Read More
In the field of medical device manufacturing, maintaining stringent quality standards is crucial. One of the key components of this process is bioburden testing and its validation. Bioburden measures the viable microorganisms on a non-sterilized surface, assessing product
Read More
Seal integrity is essential when evaluating the safety and shelf life of medical device packaging. A high-quality seal prevents microorganisms from entering through weak points, known as "channels," which can form due to various factors. These may include incomplete adhesive
Read More
In microbiology, Gram staining is more than a fundamental technique—it's an essential tool that differentiates bacteria into two major groups: Gram-positive and Gram-negative. This distinction is crucial not only for identifying bacterial species but also for supporting
Read More
My name is Grace Woods, and over the last 4 months I have worked at SteriLabs as a microbiology laboratory intern. During this time, I have been given the opportunity to explore the various testing procedures offered at SteriLabs, which has provided me with hands-on experience
Read More
The purpose is to provide guidelines and procedures for determining the microbial load present in non-sterile products. This test helps to ensure these products are free from excessive microbial contamination that could harm consumers. This test is commonly performed to check
Read More
Maintaining a clean, disinfected and organized laboratory is crucial to avoiding contamination. Aseptic technique is one of the most important practices a Microbiologist can use to minimize contamination
Read More
This blog post explores the guidelines and validation methods outlined in the "Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling, Guidance for Industry and Food and Drug Administration (FDA) Staff
Read More
In the development of a cleaning validation protocol, a critical initial phase involves the selection of an artificial test soil. This formulation aims to replicate the contaminants encountered by a medical device during clinical use
Read More
Disinfection validation confirms that the disinfection process eliminates or reduces harmful pathogens. Disinfectants used in the pharmaceutical and medical device industries must be validated for their intended use. Disinfection is a process that reduces the number of
Read More
In Canada, laboratories handling and storing Risk Group 2, 3 and 4 microorganisms are regulated by the Public Health Agency of Canada. A good day in the microbiology laboratory is when nothing “bad” happens. How do we ensure that safety is not overlooked in all the hustle of
Read More
In Canada, the regulations as overseen by Health Canada, place significant importance on the validation of cleaning processes for medical devices. This validation is a critical aspect of the manufacturing process, designed to guarantee that devices are free from contaminants,
Read More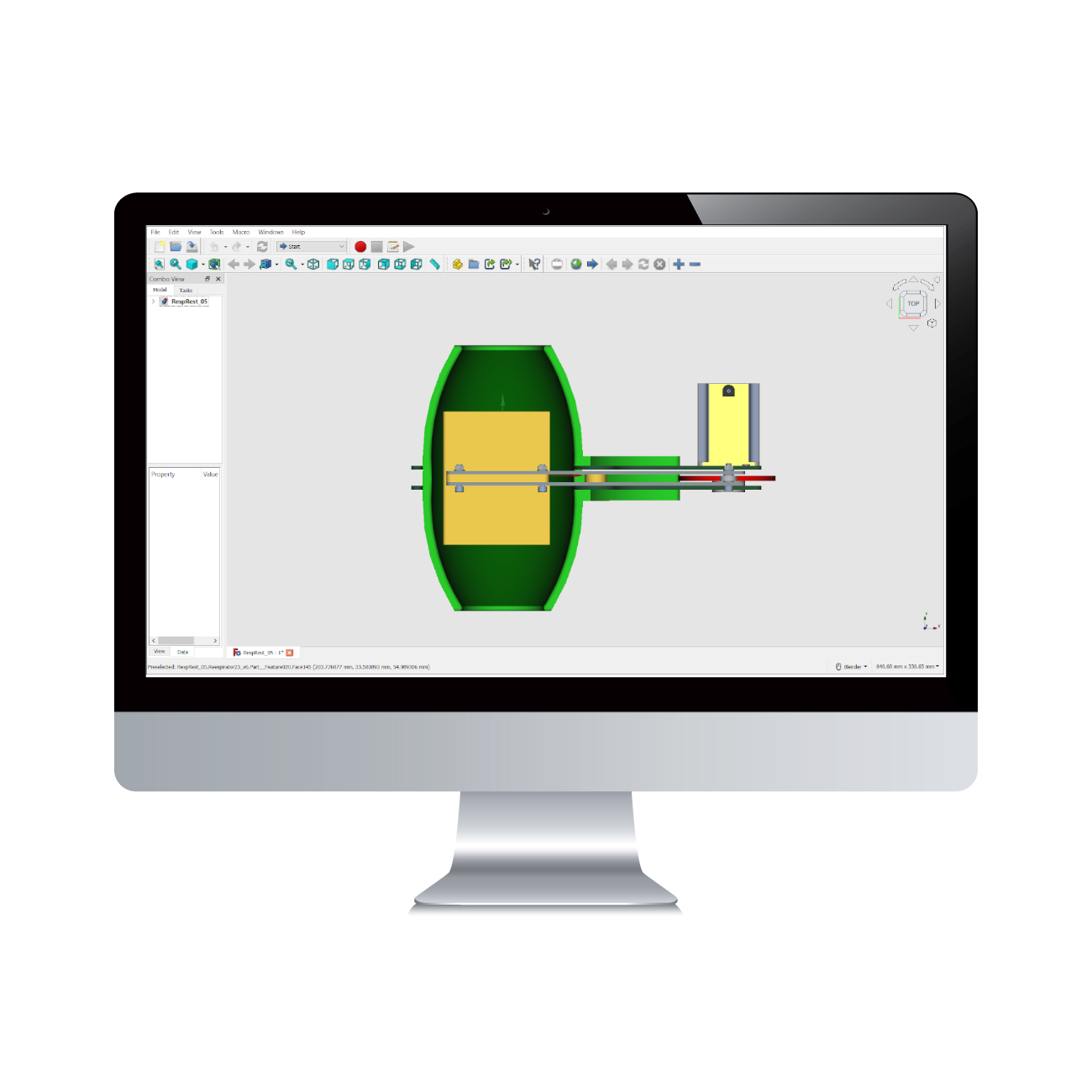
Manufacturers of medical devices are required under 21 CFR 801 to support product label claims by providing complete instructions for handling, cleaning, disinfection, testing, packaging, and sterilization, as applicable. Moreover, it is the manufacturer’s responsibility to
Read More
Ensuring the safety of these devices is critical and one main aspect of this assurance is bioburden testing that evaluates the microbial contamination on or within medical devices. In this article, we'll explore the importance of bioburden testing in ensuring the safety of
Read More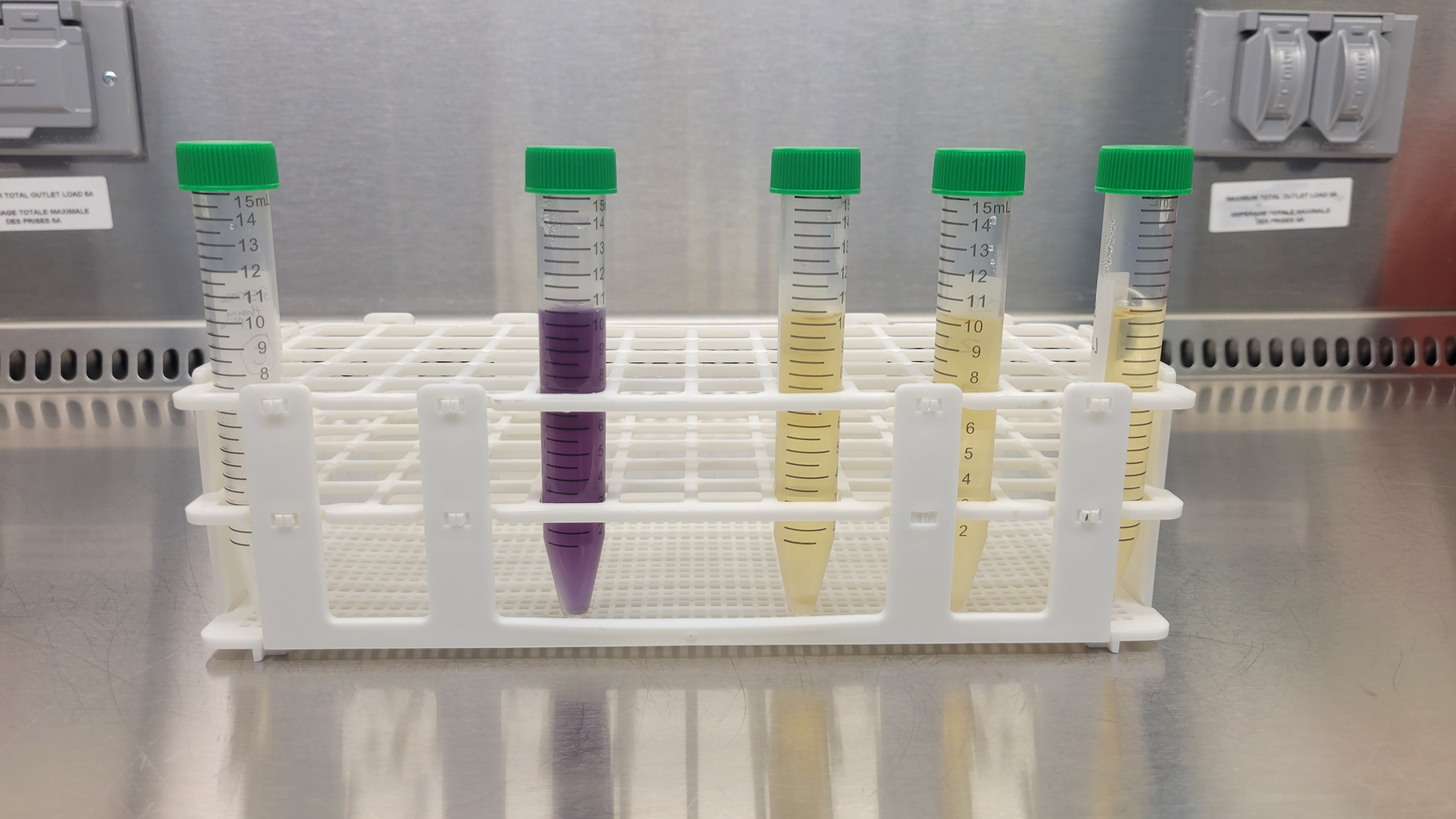
Can you imagine a scenario where a surgical instrument used during an operation had microorganisms that cause issues to the patient after the surgery? Pharmaceuticals and medical devices need to be confidently sterile. They will be used in an environment where even a small amount
Read More
In the microbiology world, culture media serves as the base of all research and discovery. These bacteria-loving recipes create the perfect environment for surviving microorganisms to grow by providing the necessary nutrients in their composition. The ingredients vary depending
Read More
For all reprocessed medical devices, the device must be sufficiently cleaned before it can go on to sterilization, as any residual contaminants remaining on the medical device can compromise the efficacy of the sterilization process
Read More
Benjamin Emmanuel completed two terms coop program at SteriLabs as a Laboratory Technician, Biochemistry. In this post, Benjamin writes about his experience for the past eight months at SteriLabs and identifies accomplishments, challenges and five keys to success in a co-op
Read More
Pharmaceuticals Sterility Testing: SteriLabs' team has successfully completed ISO 17025 accreditation audit by CALA on May 5, 2022. Accreditation to the ISO 17025 standard indicates that SteriLabs has a well-established quality management system in place to produce reliable,
Read More
In the beginning of 2020 SteriLabs and Dr. Weigert started a partnership to improve the reprocessing process through the use of highly effective medical device cleaning detergents and products
Read More
SteriLabs has been invited to present Industry Innovations at 4th International Clinical Engineering and Health Technology Management Congress hosted by AAMI, IFMBE and GCEA
Read More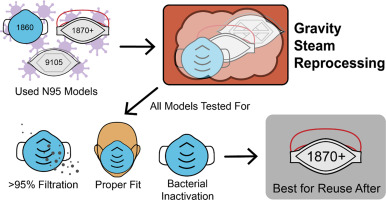
Coronavirus disease 2019 (COVID-19) has significantly impacted the health of millions of people around the world. The shortage of personal protective equipment, including N95 respirators, in hospital facilities has put frontline healthcare professionals at high risk for
Read More
Coronavirus disease 2019 (COVID-19) has significantly impacted the health of millions of people around the world. The shortage of personal protective equipment, including N95 respirators, in hospital facilities has put frontline healthcare professionals at high risk for
Read More